Real-world evidence (RWE) and other non–payer-generated data sources, such as Institute for Clinical and Economic Review (ICER) reports, provide valuable insights for formulary decision makers. Yet the use of external sources of data among payers is quite variable, according to a recent Precision Rapid PulseTM survey of pharmacy directors from health plans and PBMs.
This finding and other insights were shared during a session presentation at the AMCP Nexus 2017 Conference in Dallas last month. Key takeaways included:
- Strengths and weaknesses that payers associated with external data sources
- Emerging regulatory framework that could expand the availability and utility of external data
- Opportunities to assess and utilize RWE and other external data sources in formulary decision-making
The Rapid PulseTM findings indicate that payers are interested in data to support forecasting, total medical cost and resource offsets, and quality and outcomes performance. However, these data are not easily attainable from existing sources, often due to concerns of bias and relevance. In addition, internal obstacles, such as resource constraints, can limit the use of external data sources.
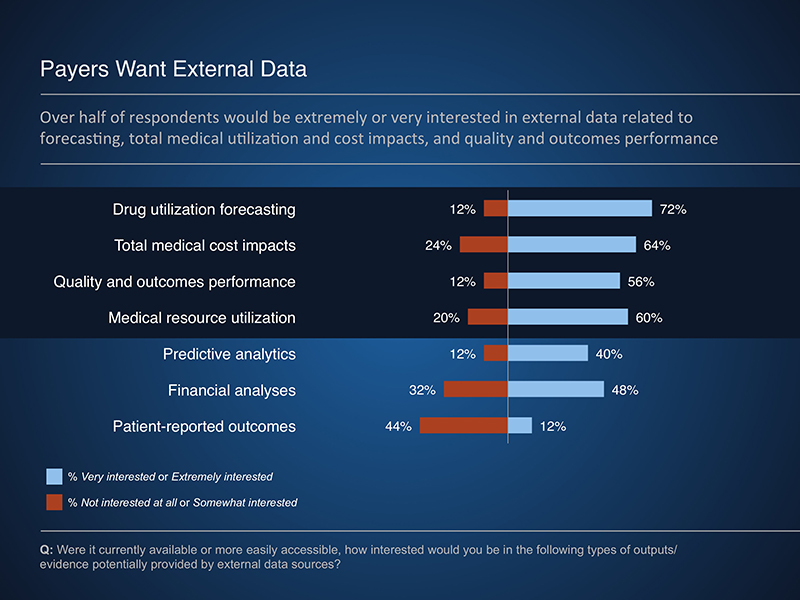
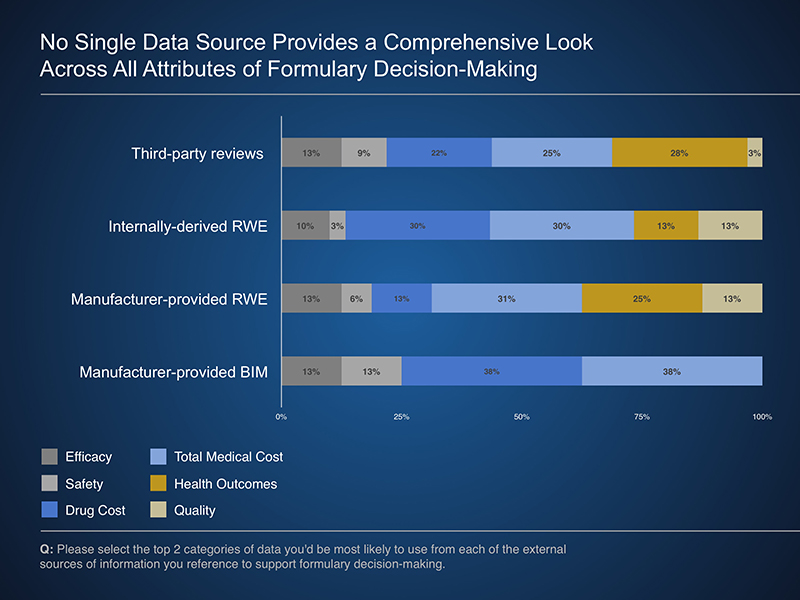
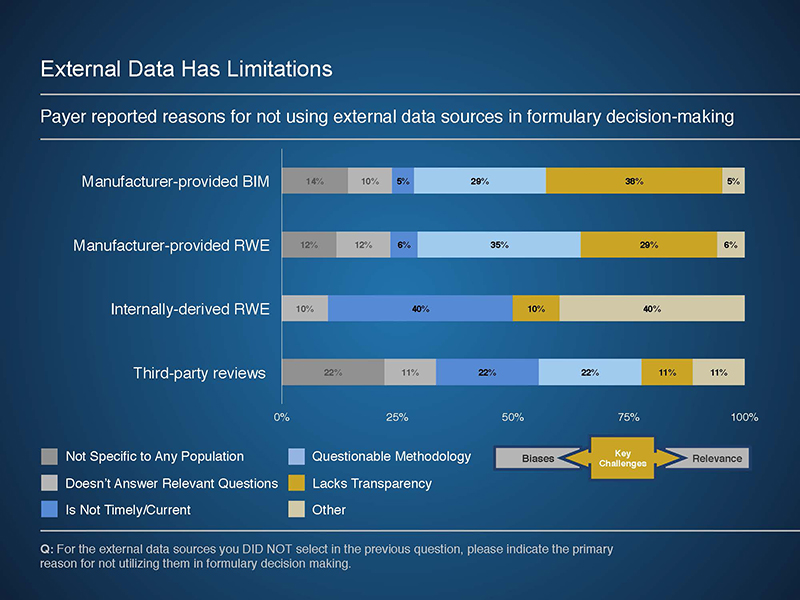
Potential applications of external data included using components of manufacturer-provided models or RWE studies to best meet the needs of a given payer. These analyses can provide valuable insights to a variety of payer programs and services, including utilization and care management program development. In addition, when provided in a transparent manner, manufacturer-provided budget impact models allow a payer to review and potentially adjust underlying assumptions to reflect their experience.
As the healthcare reimbursement landscape evolves from volume to value based, the need for evidence to support value-driven decisions will continue to grow. This session and others during AMCP highlighted multiple opportunities for both payers and manufacturers to pursue in the development and evaluation of evidence to support formulary decision-making.


